Link to our ML-Website
Designing of templates to reach the distal C-H bond:
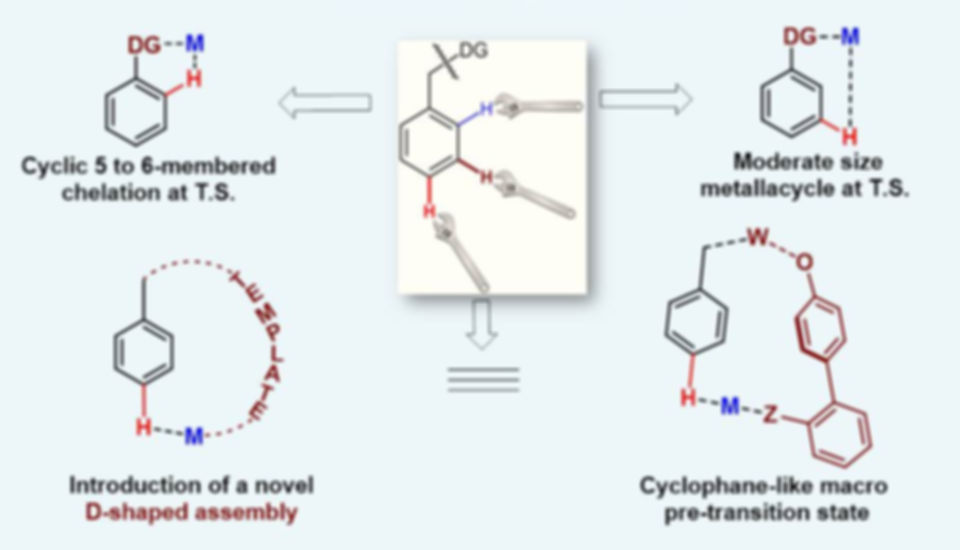
A practical protocol to simplify natural product synthesis by site selective C–H functionalization had always been a coveted target for chemists. Most often, directing group assisted metallacycle formation has served as an efficient strategy in ensuring promising regioselectivity. In this regard wide variety of ortho- functionalization stands as an archetype. Despite significant progress, directing group- assisted selective distal C–H functionalization in arenes (at meta- and para- positions) had remained an unexplored venture mainly due to the formation of a geometrically constrained metallacyclic transition state.
To address these issues, a novel class of cleavable linker with nitrile based templates that direct efficient functionalization of distal para- and meta-C–H bonds are introduced. Recently, more robust heterocycle-based directing template has been designed to deliver the various and most useful functionalizations at remote meta-position. Applicability of these template based strategies have been demonstrated by synthesizing various natural products and complex molecules through post synthetic modifications.

Aliphatic C-H functionalization:
The evolution of C–H activation/functionalization strategy has been potentially expediting the synthesis of target molecules through new disconnections in retrosynthetic analysis. By far the toughest challenge in this area is to regioselectively functionalize a particular sp3 C-H bond in alkanes. The minute reactivity differences of multitude of inert C‒H bonds and the fluxionality in aliphatic chains restricts the selective transformation at a core site within a complex molecule. Hence to incorporate functionality at a-sp3 carbon to a functional group relies on the traditional electrophilic addition or radical based reactions.
While the same at beta-carbon of carboxylic acid or gamma-carbon of amine can be performed by the assistance of directing group and transition metal. The feasibility of these reactions depends on the favored thermodynamics of the stable five membered metallacycle. However, striving for a distal sp3 C–H bond activation, other than these accessible positions, leads to more complex issues owing to thermodynamic constraints of larger metallacycle. Hence a paradigm for C-H activation reactions to functionalize more distal carbon centers is required that may impart the molecule with specific structural and functional features which would be acceptable as a lucrative molecule.
The group is currently involved in designing of more efficient of directing groups that can address the existing shortcomings and go out of stretch to offer more efficient and reliable method for distal aliphatic C–H activation at delta, epsinol and further in a selective way alongside overcoming the constitutional and conformational barriers inherent in a linear molecule. The developed protocols will open up the gates to exploit C-H activations in industries for step-economic synthesis of drugs, natural products and agrochemicals by ornamenting different molecules regioselectively, making it a versatile tool in chemist’s pocket.

Non-directed C-H functionalization:
Non-directed C–H activation represents a pioneering approach in organic synthesis, wherein specific C–H bonds within molecules are selectively activated without relying on directing groups. Non-directed C–H activation is a novel and highly advantageous method for regioselective functionalization in the current context of organic synthesis. Previously, the functionalization of C–H bonds was hindered by the necessity of directing groups to channel reactions towards desired sites. In contrast, non-directed C–H activation enables the direct transformation of inert C–H bonds into valuable functional groups, offering significant advantages in terms of step and atom economy, as well as environmental sustainability. The emergence of non-directed C–H activation has led to extensive research efforts focused on developing catalysts and methodologies. These endeavors aim to exploit the synthetic capabilities of inert C–H bonds, thereby revolutionizing organic chemistry and simplifying synthetic routes.