Link to our ML-Website

319. Near-Infrared (700–1000 nm) Photoredox Catalysis: Mechanisms, Applications and Opportunities in Organic Synthesis and Biology
Pagire, S. K.; Olivier, W. J.; Maiti, D.; Bissember, A. C.
Angew. Chem. Int. Ed., 2026, (ASAP)


318. C-H Activation Initiated Skeletal Recasting of Cyclopropane Carboxylic Acid
Maji, S.; Singh, N. K.; Manna, S.; Kabari, S.; Chauhan, R. S.; Guin, S.; Raul, A.; Gupta, P.; Maiti, D.
ACS Catal., 2026, (ASAP)


317. Ligand Design Enables Introduction of Non-Aromatic Arylating Agents in Palladium Catalyzed C-H Arylation of Arenes
Bairagi, Y.; Moni, S.; Mukherjee, S.; Maji, S.; Raul, A.; Teja, C.; De, R.; Mondal, B.; Maiti, D.
J. Am. Chem. Soc., 2026, (ASAP)


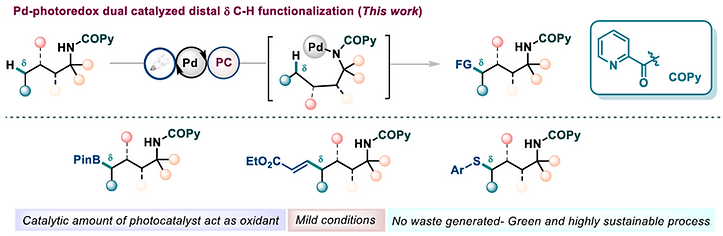
316. Auxiliary directed δ-C(sp3)‒H functionalization enabled by dual photoredox and palladium catalysis
Sinha, S. K.; Adak, A.; Maity, A.; Hawsawi, M. B.; Maiti, D.

315. Engineering Isoquinoline-derived Directing Template for Distal C5-H Functionalization of Bicyclic Aza-arenes
Mandal, A.;† Bano, R.;† Chattopadhyay, S.; Banerjee, R.; Borah, D.; Gupta, N.; Shanmugam, M.; Maiti, D.
Angew. Chem. Int. Ed., 2025, e23983


314. A general strategy to access pharmaceutically relevant pyridines from nitroarenes
Saha, A.; Pan, A.; Ghosh, D.; Pal, A.; Guin, S.; Redhu, A. K.; Gota, V.; Maiti, D.
Angew. Chem. Int. Ed., 2025, e17867


313. A Generalized Approach for Distal C–H Arylation of Organic Building Blocks: Unveiling the Role of Counter Anion
Grover, J.;† Prakash, G.;† Mandal, A.;# Ghosh, D.;# Maiti, S.; Empel, C.; Maiti, D.


312. A metal-catalyzed triplet relay strategy for aza-Pauson-Khand reactions
Phama, Q. H.; Sliusarevskyi, I.; Saha, A.; Kloene, L.; Linnartz, N. J.; Empel, C.; Oppel, I. M.; Maiti, D.; Koenigs, R. M.
Chin. Chem. Lett., 2025, 112306


311. Pd-catalyzed distal dehydrogenative δ-arylation and γ-lactonization of acyclic free carboxylic acids
Ghosh, A.; Chauhan, R. S.; Das, N.; Ali, W.; Maiti, D.


310. Facile oxidative amination with imidazole and L-histidine coordinated cobaloximes
Singh, R.;† Panja, S.;† Pal, B.; Manna, P.; Nandi, C.; Ghorai, S.; Dutta, A.; Maiti, D.
Chem. Commun., 2025, 61, 14927


309. Metallaphotoredox-Catalyzed Cross-Electrophile Couplings of Aryl Chlorides and Alkyl Halides: Harnessing the s-Donor/π-Acceptor Synergy of a 2-(1H-Imidazol-2-yl)pyridine Ligand
Ghosh, P.; Maiti, S.; Gunawan, N.; Pal, B.; Ghosh, A.; Bissember, A. C.; Maiti, D.
Angew. Chem. Int. Ed., 2025, e202509809


308. Spatially Tweaked Amide Directing Group Enables High-Turnover Pd Catalysts for Site-Selective Remote C−H Arylation
Sivaraj, C.; Gandhi, T.; Maiti, D.


307. Consecutive multiphoton mediated defluorinative amination of fluoroarenes
Saha, A.;† Roy, M.;† Maji, S.; Rana, G.; Maiti, D.; Adhikari, D.
J. Am. Chem. Soc., 2025, 147, 20735


306. Scalable photoinduced cycloaddition for synthesis of biorelevant oxazoles
Saha, A.; Bianchi, M.; Casali, E.; Maiti, D.


305. Harnessing C–H acetoxylation: a gateway to oxygen-enriched organic frameworks
Grover, J.; Dutta, B.; Ghosh, D.; Shee, P. K.; Maiti, S.; Werz, D. B.; Maiti, D.


304. Metal-catalysed non-directed C(sp2)–H bond activation
Agrawal, S. K.;† Porey, S.;† Bairagi, Y.;† Maiti, S.; Bissember, A. C.; Maiti, D.
Chem. Soc. Rev., 2025, 54, 6122


303. Copper(II) triflate/Vitamin-C Promoted Modified Morita-Baylis-Hillman Annulations: A Facile Route to Fluorescent Cyclopentene Derivatives
Azad, S. A.; Barik, P.; Samanta, J.; Sepay, N.; Sebastian, A. T.; Ghara, S.; Giri, S.; Molla, M. R.; Maiti, D.; Samanta, S.
Chem. Eur. J., 2025, 31, e202501040


302. Synthesis of Lactones and Lactams by C(sp3)–H bond Functionalization
Premkumar, E.; Sreedharan, R.; Ghosh, P.; Pal, T.; Maiti, D.; Gandhi, T.
Chem. Soc. Rev., 2025, 54, 6238


301. Remote meta-C−H α-Fluoro-alkenylation of Arenes Using gem Difluoroalkenes
Bairagi, Y.;† Porey, S.;† Mahato, I.; Maiti, D.