Link to our ML-Website
Bio-inspired catalysis :
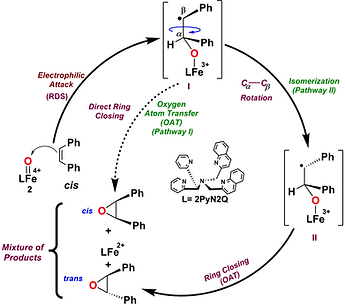
Halogenations occurs during biosynthesis of more than 4000 natural products that display biological activity of pharmacological interest including anticancer, antibacterial, antiviral, antifungal, and anti-inflammatory activities. Chlorination is the predominant modification in nature, followed by bromination and iodination. Enzymes such as myeloperoxidase (MPO), chloroperoxidase (CPO), bromoperoxidase (BPO), α-ketoglutarate dependent halogenase and FADH2 dependent halogenase carry out halogenation reactions during natural product synthesis. These enzymatic halogenation reactions occur either via a radical (Cl) or via an electrophilic (hypohalide) (ClO-) mechanism.
Heme peroxidases (MPO, CPO) catalyze oxidation of Cl- to ClO- via formation of a transient hypochloritoiron(III)porphyrin intermediate which is responsible for the electrophilic halogenation reactions. In case of non-heme iron enzymes like α-ketoglutarate dependent halogenase and FADH2 dependent halogenase, a halide radical rebound mechanism has been proposed. . We have developed metal mediated decarbonylative halogenations by vanadium oxoperoxo species and direct halogenation by non-heme biomimetic iron-oxo complexes. Development selective aliphatic and aromatic halogenations over hydroxylation by using non-heme 1st row-transition metals complexes are our research goal.